Abstract
Background:
Infections remain a major cause of morbidity and mortality in individuals with multiple myeloma. These individuals show a suboptimal response to pneumococcal polysaccharide vaccine (PPV23). Pneumococcal conjugate vaccine (PCV13) elicits a T cell dependent response and may lead to greater immunogenicity, and is therefore the recommended vaccine in patients with multiple myeloma. This study compares the response to PCV 13 vaccine in patients with multiple myeloma to healthy controls.
Methods:
Eligible patients received PCV 13 vaccine on day 1. Streptococcal pneumonia serotype IgG titers were drawn at baseline, day 30, (+/- 7 days) and day 180 (+/- 30 days). Given the small sample size, Fisher's exact test was used to analyse odds ratio significance for the 2 X 2 contingency tables. The type I error rate was set at less than 0.05. The odds ratio confidence intervals were derived from the log odds ratios using standard methods. SAS® University Edition was used for analysis.
Results:
There were 7 patients with multiple myeloma and 18 healthy controls enrolled for the study. The mean age was 60 years for the multiple myeloma group, versus 68.5 years for the healthy controls. Individual serotype response was defined as the following:
If < 1.3, need to increase 2 fold to above 1.3 OR increase 4 fold
If > 1.3, need to increase 2 fold
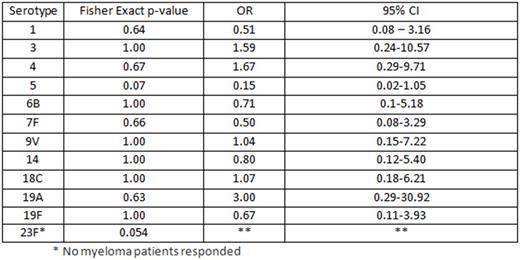
Response to 12/13 IgG serotypes are reported in the table attached.
Conclusions:
Vaccination with PCV13 produced a similar response in patients with multiple myeloma compared to healthy controls. A larger study may be needed to confirm these findings and find the long term clinical benefits of vaccination with PCV13 in patients with multiple myeloma.
Mustafa: Shire: Research Funding; Genentech: Speakers Bureau; Teva: Speakers Bureau; Greer: Speakers Bureau. Jamshed: TAKEDA pharmaceuticals: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal